Explain How Liquid Chromatography Separates Compounds of Different Polarity
By creating an account on LiveJournal you agree to our User Agreement. All of these are true of Newtons third law of motion except.

Principles Of Chromatography Stationary Phase Article Khan Academy
Paper chromatography is a method that is used for testing the purity of compounds and the identification of substances.

. Because R f values are standard for a given compound known R n values can be used to aid in the identification of an unknown. R f values are calculated by dividing the distance the pigment travels up the paper by the distance the solvent travels the solvent front. When a soda water bottle is opened the pressure inside the bottle decreases.
Graham Solomons Craig B. The means vary enormously and probably assess different functional components of the barrier. This glossary of chemistry terms is a list of terms and definitions relevant to chemistry including chemical laws diagrams and formulae laboratory tools glassware and equipmentChemistry is a physical science concerned with the composition structure and properties of matter as well as the changes it undergoes during chemical reactions.
However none of these can detect and monitor the real-time concentration fluctuation of. Depending on context the term may or may not include ions which satisfy this criterion. Fryhle Scott A.
Must contain at least 4 different symbols. Partition chromatography PC follows the liquidliquid extraction principle based on the relative solubility in two different immiscible liquids. In the kinetic theory of gases the term molecule is.
It is 26 editions old. When two objects interac Draw out the major differences between outcomes based education OBE and current curriculum and Assessment. It has been used by more than 2 million students.
Study Guide and Solutions Manual to Accompany TW. Paper Chromatography Apart from all different methods of separation of types of a mixture there is a special technique for separation and. Principles involved in chromatography method development especially for the analytical method development for the separation identification purification and quantitative estimation of organic compounds using the liquid chromatography techniques HPLC UPLC LCMS preparative HPLC etc were emphasized in this chapter.
The disadvantage of an easily. Preface The Essentials of Physical Chemistry has been written for BSc students. The barrier assessments are further hindered by the natural variability of this functional entity depending on species and genes as well as on diet.
Not based on your username or email address. Snyder Jon Antilla. In quantum physics organic chemistry and biochemistry the distinction from ions is dropped and molecule is often used when referring to polyatomic ions.
The solvents for the TLC plate can be changed easily and it is possible to use several different solvents depending on your desired. As in most other forms of chromatography paper chromatography uses R n values to help identify compounds. In the fourth part the means to assess intestinal permeability are presented and critically discussed.
Difference in solubility of a gas in the liquid at different pressures Eg separation of dissolved O2 by heating water. Only month and day are displayed by default. Therefore once the best solvent is found it can be applied to other techniques such as High performance liquid chromatography.
Separations in the paper chromatography method involve the partition principle. More than 1 compound can be separated on a TLC plate as long as the mobile phase is preferred for each compound. In the method of paper.
It really has been that long. Or you can use social network. A molecule is a group of two or more atoms held together by chemical bonds.
CO2 gas fizzes out of the bottle. It has been national bestseller for more than 65 years. At least 1 number 1 uppercase and 1 lowercase letter.
The paper chromatography method is a useful technique due to the reason it is relatively quick and needs only small quantities of material. It features an extensive vocabulary and a. Be sure to include hydrogen bonding polarity.
In the early stage one liquid phase was coated to a solid matrix silica gel carbon cellulose etc as the stationary phase and another liquid phase was employed as the mobile phase. The traditional methods for biomolecule identification and characterization mainly focus on ultraviolet-visible absorption spectroscopy mass spectrometry gasliquid chromatography and immunological methods such as enzyme-linked immunosorbent assays. Predicts what occurs to the forces when one object comes in contact with another object.

Principles Of Chromatography Stationary Phase Article Khan Academy

Principles Of Chromatography Stationary Phase Article Khan Academy

Principles Of Chromatography Stationary Phase Article Khan Academy
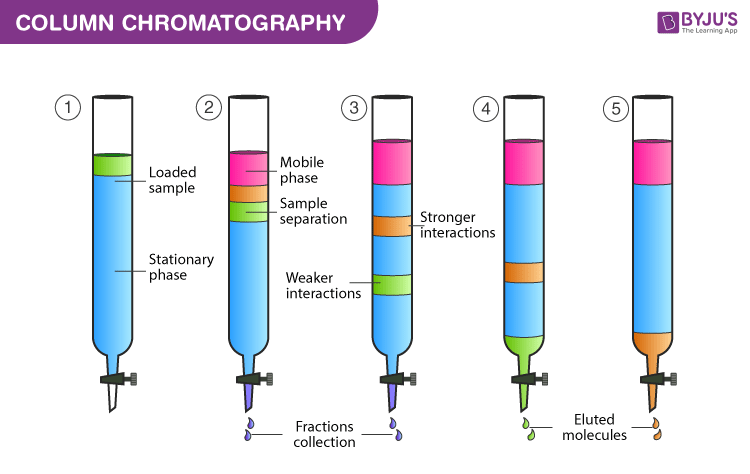
Column Chromatography Principle Procedure Applications Elution In Chromatography
0 Response to "Explain How Liquid Chromatography Separates Compounds of Different Polarity"
Post a Comment